Below are the tutorial movies for learning how to correctly draw atomic orbital configurations (electron configurations) for the electrons in atoms and ions. Read the directions on the right.
This movie may also be viewed in iTUNES.
AO (Atomic Orbital Configuration)
Part 1 - Drawing Electron Configurations of Lithium, Carbon & Nitrogen Atoms

Part 2 - Drawing the Electron Configuration for a Phosphorus Atom

Part 3 - Drawing the Electron for Iron metal

AO Configuration for Ions
Part 5 - Electron Configuration for Sodium Ion

Part 5 - Electron Configuration for Chloride Ion

Part 3 - Electron Configurations for Transition Metals
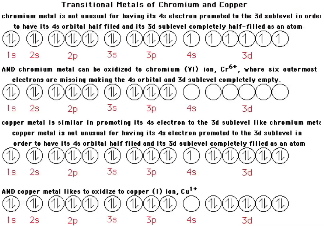
Atoms chemically will lose, gain or share electrons to achieve filled, half filled or empty outermost orbitals and/or to become isoelectronic its closest noble gas neighbor.