Schrodinger's Model - Electron Configuration for Ions, Cations & Anions (Part 3)
Below are tutorials movies for learning how to correctly draw electron and orbital configurations ions, anion and cations in the periodic table.
Read all directions on the right and view all linked resources ==>
Scroll downward & click on the topic link or thumbnail image to view each resource. Please have patience while they download. These resources may have LARGE file size. A captioned playlist is linked at the bottom of this page.
| To view this page in a web browser with frames (click here) |
MANTRA:
|
Part 1 - Electron Configuration for Sodium Ion
Part 2 - Electron Configuration for Chloride Ion

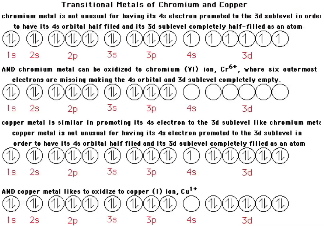
Part 3 - Electron Configurations for Transition Metals
Part 4 - Continue to Periodic Trends - Evidence to Support Atomic Theory
| Captioned Playlist of all videos to open in new window (click here) |
Overall Review for Bohr and Schrodinger |