VSEPR Theory & Molecular Geometry Atoms of C, N, O
How many atoms does it take to produce a geometric angle??? ANSWER: Always three (3) atoms.
- The orientation of objects in space—visualizing spatial orientations in our mind's eye—can be a mental struggle.
![]() IMPORTANT: Study the animation, noting the important symbolism shown below:
IMPORTANT: Study the animation, noting the important symbolism shown below:
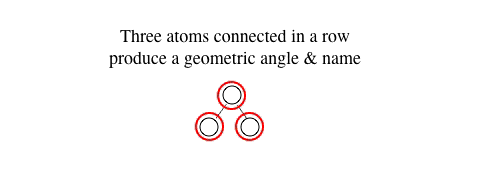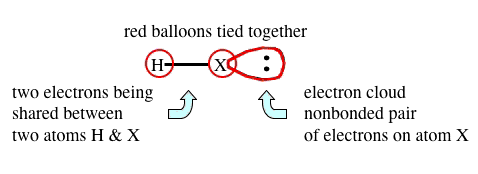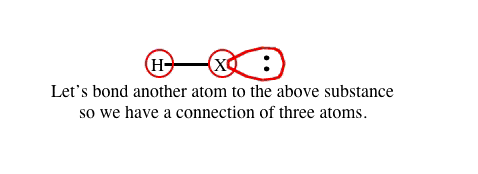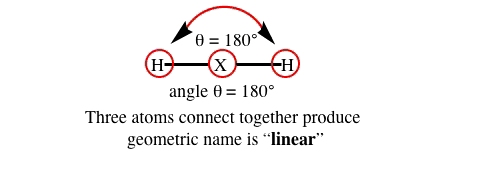
- Note the "
 " represents electrons being shared between two atoms, and
" represents electrons being shared between two atoms, and - This image
 is used as our representation showing an electron cloud for non boned electrons about an atom.
is used as our representation showing an electron cloud for non boned electrons about an atom. - Nonbonded pairs of electrons are NOT being shared between two atoms.
![]() When visualizing atoms and their spatial orientation in a substance, remember these things known to be true:
When visualizing atoms and their spatial orientation in a substance, remember these things known to be true:
1) the surface area of atoms and electrons are negatively charged,
2) The Law of Electrostatics - "like charges repel like charges" and seek the greatest degree of separation, and
3) three atoms are required to produce an angle & geometric "named" identifier about a central atom.
Applying the Law of Electrostatics, opposites attract and like charges repel, is important in our understanding of VSEPR theory–an empirical theory.
- Click 'Next' to continue to learn more about VSEPR theory needed in identifying molecular geometry about individual atoms in molecules.
Your are responsible for all content—tutorials, videos, powerpoints, PDFs & drill and practices—presented in these linked resources associated with these directions. Please click the 'Next' button to continue the learning process.
|