Chemists look at matter's composition, form and structure deciding how and if it can be physically separated and chemically changed.
- Generally, as chemists & Citizens of Science, our approach to understanding matter begins by listing all physical observations on the system being observed.
- Believe it or not, in some ways we are already trained to think like a chemist since we make physical & empirical observations everyday.
- It's okay to use your imagination. There is NO Comprehension without PICTURING and in this course we'll be drawing lots of pictures, and your comprehension& critical analysis will at times be assessed as to whether you comprehend the picturing & imagery learned in our course.
Let's make some general statements about the imagery on a handout linked below. |
Comprehension with PICTURING

an atom  versus a molecule versus a molecule 
(s) = solid; (l) = liquid; (g) = gas
 NOTE: hyperlinks in PDFs may or not work and/or appear when viewing the handout from a phone, ipad or tablet. Always work from a reliable computer. NOTE: hyperlinks in PDFs may or not work and/or appear when viewing the handout from a phone, ipad or tablet. Always work from a reliable computer.
|
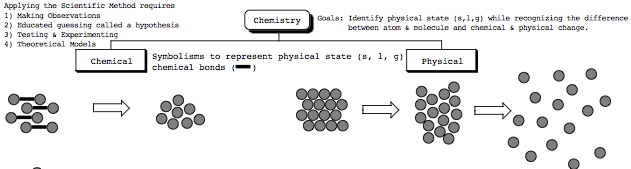 <=== roll your mouse cursor over this picture shown on the left to view "Physical vs Chemical Empirical Observations" handout. <=== roll your mouse cursor over this picture shown on the left to view "Physical vs Chemical Empirical Observations" handout. - The goal of the handout is to help us comprehend the differences between physical state (s,l,g) and the chemical differences between an atom
 & molecule & molecule  through picturing. through picturing.
- Working from a reliable computer, the handout is available (click here) and will open in new window as a "Drill & Practice."
 This a very important handout, be sure to study all that is on this handout This a very important handout, be sure to study all that is on this handout- For the images on the handout, see if you can identify physical state (s,l,g) recognizing the difference between an atom & molecule, and physical and chemical change.
- Hypothesize why there are different spatial orientations and representations being shown for each type of physical state: solid, liquid & gas?
- Using the handout as a "Drill & Practice"
- Click back and forth between the questions and answers to get a better sense for the differences between physical and chemical properties.
- Prepare of list of physical terms (i.e., boil) and a list of chemical terms (i.e., decompose) for each transformation.
Prepare a list of empirical observations about what you are observing on the handout. This will give you additional practice recording physical observations. Then mentally answer the following questions:
- Can you summarize important physical and chemical ideas these images on the handout are representing?
- Can our images be divided in to physical and chemical parts?
- Are you able to compare and contrast any similarities and differences between physical and chemical ideas using the picturing, imagery and symbolism shown?
- You are going to be assessed on whether you comprehend and understand the content on this page.
- Please email me if you have any questions.
- Then, press the 'Next' button below to continue the learning process.
|
![]()
