43. What determines the chemical reactivity of an element?
The number of electrons left in the outer most shell. Remember that the number of electrons is equal to the number of protons in the nucleus of the atom.
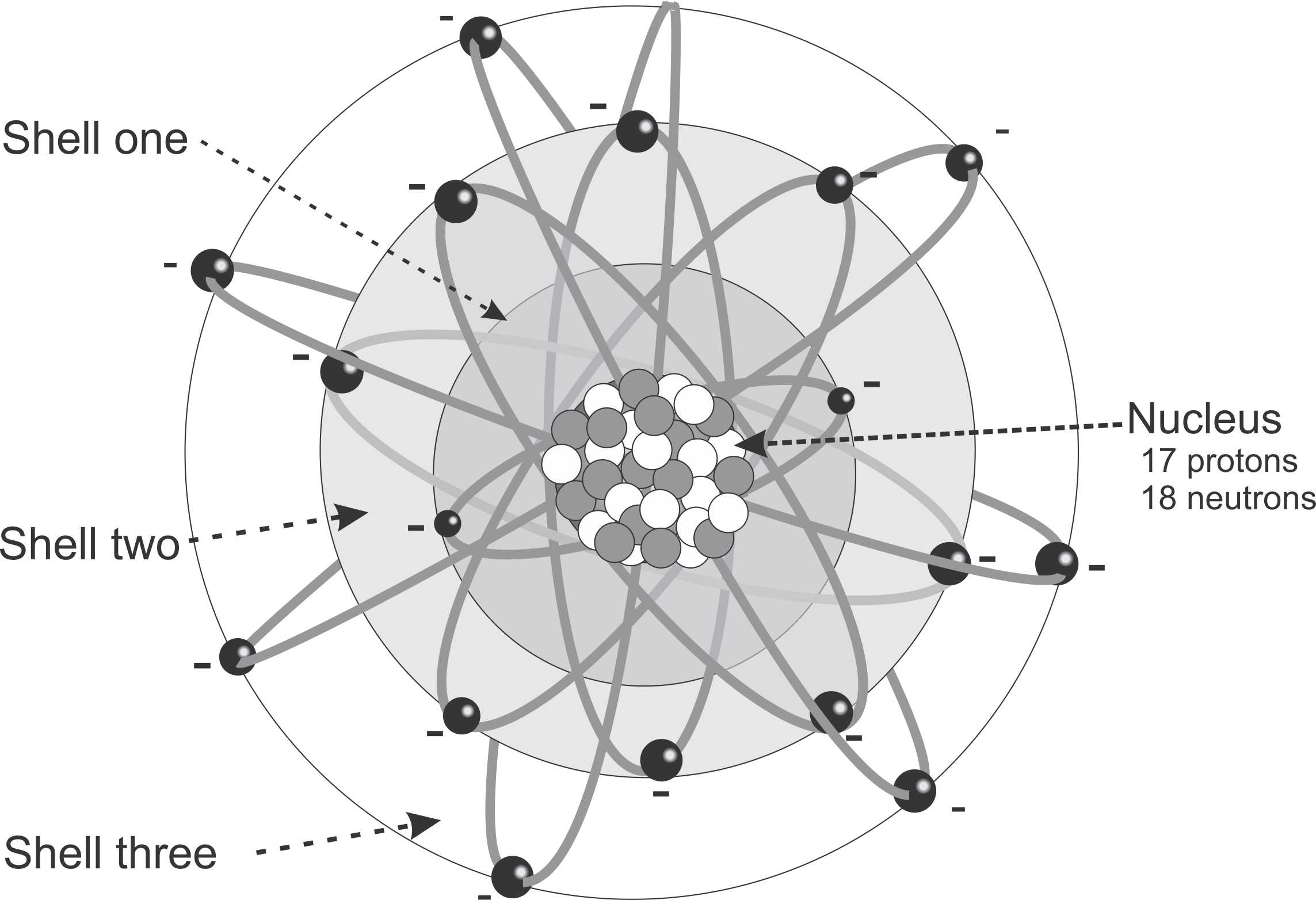
This illustration has 17 protons therefore it has 17 electrons. The 17 electrons (octet rule) are distributed with two electrons in the first shell, eight electons (in four orbits) in the second shell and seven electrons in the third shell. The chemical reactivity would be determined by the seven electrons. In general this atom would gain one electon from another atom to eventially have 8 electrons , but would become negatively charged because it would have one more negative charge (18 electrons) than positive charge (17 protons).
If this atom had only one electron in the outermost shell, it would tend to lose it to another atom becoming positively charge with one less electron than protons.