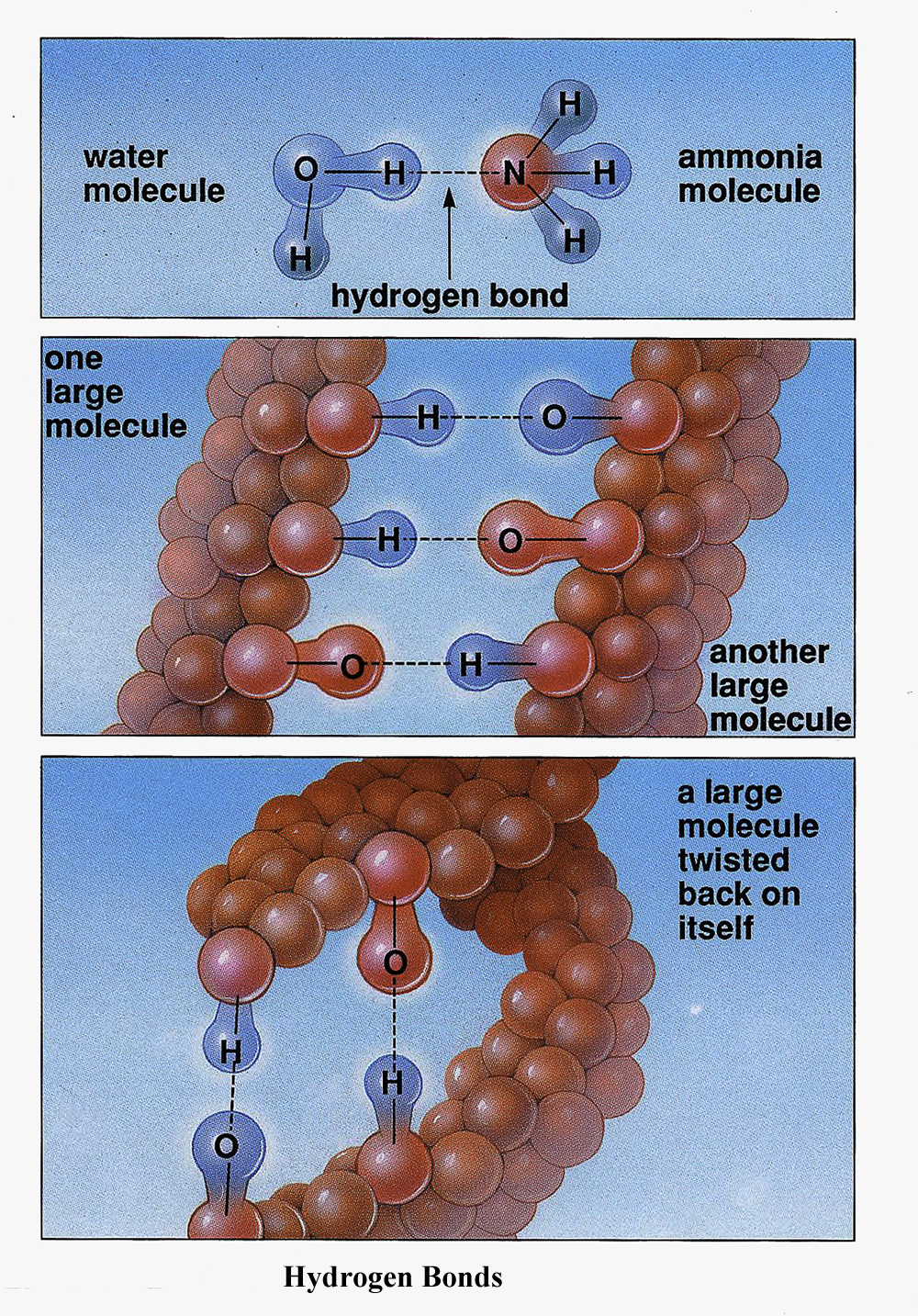
9. Define a hydrogen bond.
The hydrogen only occurs between different molecules. A hydrogen attached to a Carbon or Oxygen is not considered a hydrogen bond. When a hydrogen is attached to a larger atom such as nitrogen and oxygen, the hydrogen's electron is weakly pulled toward the larger atoms. This results in the larger atom gaining a weak negative charge and the hydrogen gaining a weak positive charge. This weak positive of the hydrogen will attract other weakly negatively charged atoms of oxygen and nitrogen on another molecule. This will result in complex multiple bonding in large organic molecules.